Ordering
*link will take you to our exclusive distribution partner site
*link will take you to our exclusive distribution partner site
Highly quality dNTPs are a vital requirement for successful PCR, as the presence of contaminating impurities will result in a decrease in amplification sensitivity and product yield. Meridian ultra-pure dNTPs undergo highly stringent purification steps to give a greater than 99% purity and are tested or the absence of DNase, RNase, Protease, Nickase activity. They are then quality controlled& in a variety of applications, ensuring that they are highly suitable for the most sensitive of PCR techniques.
All Meridian dNTP Mixes are supplied as an equimolar solution of ultra-pure dATP, dCTP, dGTP and dTTP as lithium salts. Lithium salts have greater resistance to repeated freezing and thawing cycles than sodium salts and remain sterile due to the bacteriostatic activity of lithium towards various microorganisms, giving greater reliability and an extended shelf life.
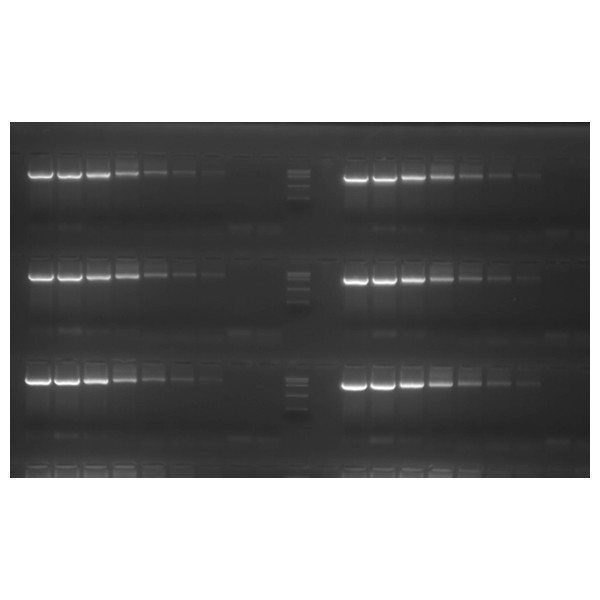
Fig. 1 Multiple rounds of freeze-thawing
Two different batches of dNTPs were freeze-thawed 5, 10 and 20 times and two-fold serial dilution was used in a PCR reaction. Both batches produced identical PCR products of 2 kb whether freeze-thawed 5 times or freeze-thawed 20 times.
Fig. 2 Long-term storage
Long-term stability of dNTPs was determined on dNTPs stored at -20oC for one year and two years. A two-fold serial dilution of these dNTPs was used in a PCR reaction to produce identical PCR products regardless of age of the dNTPs.
| Reagent | BIO-39044 | BIO-39053 | BIO-39043 | BIO-39028 | BIO-39029 |
| dATP, dCTP, dGTP, dTTP (10 mM) | 1 x 1 mL | 10 x 1 mL | - | - | - |
| dATP, dCTP, dGTP, dTTP (40 mM) | - | - |
1 x 500 μL |
- | - |
| dATP, dCTP, dGTP, dTTP (100 mM) | - | - | - |
1 x 500 μL |
4 x 500 μL |
All kit components should be stored at -20°C upon receipt for optimum stability. Repeated freeze/thaw cycles should be avoided. For long-term storage, aliquoting is recommended.
When stored under the recommended conditions and handled correctly, full activity of the kit is retained until the expiry date indicated on the outer box label.
On Dry Ice or Blue Ice.
The standard concentration of each dNTP in a PCR reaction is 0.2 mM. If the starting stock is a 100mM solution of each dNTP, you need to add 0.1 µl of each nucleotide to a 50 µL standard PCR reaction. Since this is not convenient, it is recommended to prepare mixes: If the 100 mM dNTP stock solutions are mixed in equimolar amounts, the concentration of the mix will be 100mM total or 25 mM of each nucleotide. From a 100x stock, you need to add 0.5 µL to a 50 µL reaction. Meridian also offers more diluted mixes of 40mM total (10mM each), which is a 50x stock solution and 10 mM total (2.5 mM each) 10x working stock. Several different dNTP Mix formats are available from Meridian as ready-to-go solutions.
Your PCR assay can be dramatically affected by a dNTP preparation containing inhibitors, which have resulted from an inadequate manufacturing process. Several parameters must be taken into account when purity is sought and each dNTP should preferably be free of ribonucleoside triphosphates, other dNTPs, modified nucleotides (methylated, deaminated etc.), deoxynucleoside di- and monophosphates (dNDPs and dNMPs), heavy transition metals, inorganic pyrophosphates (PPi) and nucleoside tetraphosphates.
Yes. The optimal pH for storage of nucleotides is from pH 7.5-8.2 (pH at 20°C). An acidic pH will cause hydrolysis of dNTPs to dNDPs and dNMPs, rendering them less suitable for PCR applications. During freezing/thawing cycles, the pH of the dNTP solutions can differ from the pH at 20°C. The pH for lithium salt solutions is not as temperature-dependent as with sodium salts, hence where lithium salts are used, no dramatic shifts in pH occur when dNTPs are repeatedly frozen and thawed. This results in the dNTP preparation being more stable and, consequently, having a much longer shelf life than with sodium salts.
The enzymatic synthesis of dNTPs uses highly specific enzymatic systems which eliminate impurities and PCR inhibitors, such as modified nucleotides, PPi and deoxynucleoside tetraphosphates. PCR reactions are impeded by the presence of contaminants resulting from chemical manufacturing processes, such as traces of dNDPs, pyrophosphates or other ionic species (e.g. acetate). Such contamination may lead to poor yields or to no PCR product at all. Unless thoroughly purified, chemically synthesized dNTPs often contain deoxynucleoside tetraphosphates which are powerful PCR inhibitors. Chemical synthesis can also lead to deamination and other nucleotide modifications whereas enzymatic synthesis of dNTPs bypasses these risks.
Reference was made earlier to the greater solubility of dNTPs in lithium salts than in sodium salts. Also, dNTPs presented in lithium salts are more resistant to repeated freezing and thawing than those presented in sodium salts. Furthermore, they remain sterile during the entire storage period (the lithium ion has been shown to have significant bacteriostatic activity towards various microorganisms). Finally, using lithium-salt nucleotide preparations reduces salt-induced artifacts and increases the legibility of sequencing gels. Lithium salts are highly suited to PCR sequencing and labeling applications.