Ordering
*link will take you to our exclusive distribution partner site
*link will take you to our exclusive distribution partner site
BioMix™ is a complete, ready-to-use, 2x reaction mix containing ultra-stableTaq DNA polymerase. BioMix allows you to perform PCR assays of many common genomic and cDNA templates by simply adding water, template and primers.
BioMix™ is a complete ready-to-use 2x reaction mix containing a stable Taq DNA polymerase. Developed to perform PCR assays of many common genomic and cDNA templates, simply add water, template and primers.
BioMix reduces the time required to set up reactions, thereby minimizing the contamination risks. Greater reproducibility is ensured by reducing the number of pipetting steps that can lead to errors.
BioMix is supplied with additional MgCl2 solution that allows fine-tuning of reactions, if required.
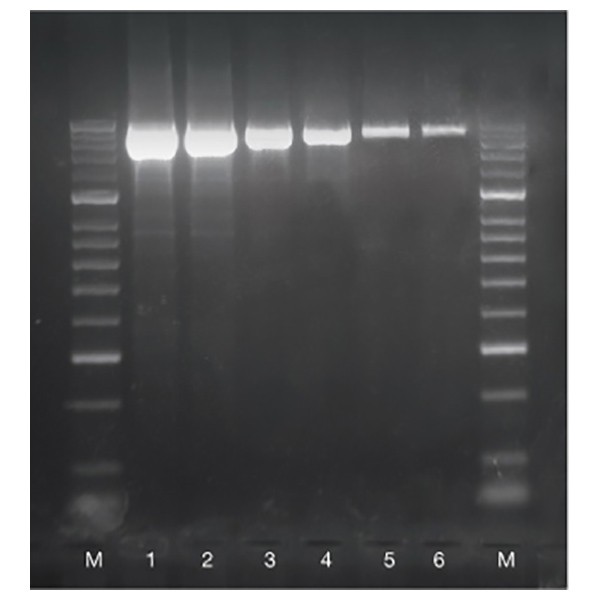
Fig.1 Sensitivity of BioMix
A two-fold serial dilution of mouse genomic DNA from 50 ng (lane 1) to 1.5 ng (lane 6) of a 1.8 kb fragment of the rn18s gene was amplified, to show the sensitivity of BioMix.
|
Reagent |
500 Reactions |
|
BioMix™ |
10 x 1.25 mL |
|
50 mM MgCl2 Solution |
1.2 mL |
2x
All components are shipped on dry/blue ice and should be stored at -20°C upon receipt for optimum stability. Repeated freeze/thaw cycles should be avoided. When stored under the recommended conditions and handled correctly, full activity of the reagents is retained until the expiry date on the outer box label.
Components may also be stored at +4°C if required, although storage at -20°C is recommended. When stored at +4°C, reagents will remain stable for a period of up to two weeks from date of receipt.
Meridian's mixes contain magnesium chloride at the following concentrations (please see table below). An additional tube of 50 mM MgCl2 solution is supplied with each mix to facilitate further optimization if required.
| Meridian Mix | Final Magnesium Concentration |
| ACCUZYME Mix | 2.0 mM. |
| BioMix / BioMix Red | 2.5 mM. |
| ImmoMix / ImmoMix Red | 3.0 mM. |
| BIO-X-ACT Short Mix | 2.0 mM. |
| MangoMix | 2.5 mM. |
| MyTaq | 3.0 mM. |
| RANGER | 1.5 mM. |
| Observation | Recommended Solution(s) |
| No or low PCR yield | Enzyme concentration too low – increase the amount of enzyme in 0.5 U increments. |
| Primers degraded – check quality and age of the primers. | |
| Magnesium concentration too low – increase concentration in 0.25 mM increments with a starting concentration of 1.75 mM. | |
| Primer concentration not optimized. Titrate primer concentration (0.3-1 µM); ensuring that both primers have the same concentration. | |
| Template concentration too low – Increase concentration of template. | |
| Perform a positive control to ensure that the enzyme, dNTPs and buffers are not degraded and/or contaminated. | |
| Multiple Bands | Primer annealing temperature too low. Increase annealing temperature. Primer annealing should be at least 5°C below the calculated Tm of primers. |
| Prepare master mixes on ice or use a heat-activated polymerase. | |
| For problems with low specificity. Try adding 3% DMSO (not supplied) to improve specificity. | |
| Smearing or artifacts | Template concentration too high. Prepare serial dilutions of template. |
| Too many cycles. Reduce the cycle number by 3-5 to remove non-specific bands. | |
| Enzyme concentration too high - decrease the amount of enzyme in 0.5 U increments. | |
| Extension time too long. Reduce extension time in 0.5-1 minute increments. |