Ordering
*link will take you to our exclusive distribution partner site
*link will take you to our exclusive distribution partner site
IMMOLASE™ is a heat-activated, thermostable DNA polymerase. IMMOLASE provides high yield and improved specificity when compared to standard polymerases and can eliminate the presence of non-specific binding, such as primer-dimers and mis-primed products.
IMMOLASE™ is a heat-activated thermostable DNA polymerase and provides high yield and improved specificity when compared to standard polymerases. IMMOLASE can eliminate the presence of non-specifics such as primer-dimers and mis-primed products.
IMMOLASE is inactive at room temperature and requires activation by heat treatment for 10 minutes prior to PCR cycling. This provides greater flexibility in reaction set ups and allows the premixing of PCR reagents at room temperature. Subsequently, the reaction can be handled according to preferred protocols for thermostable DNA polymerases.
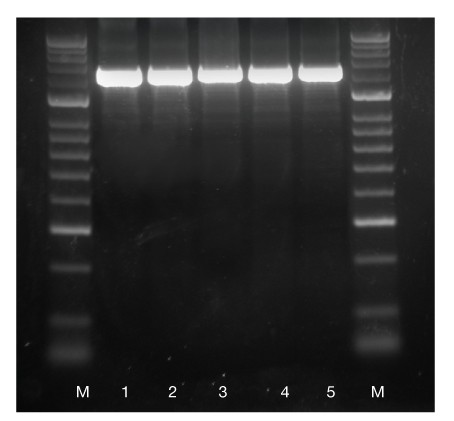
Fig. 1 Extremely high yield amplification
A 1.4 kb mouse rn18s gene fragment was amplified with 2.5 units of IMMOLASE DNA Polymerase (lanes 1-5). The rn18s fragment was amplified from 100 ng of mouse genomic DNA. The PCR was performed in 50 μL reaction mixtures containing 1.5 mM MgCl2. HyperLadder 50bp (M). Extremely high yield is achieved with every replicate.
Fig. 2 Illustration of IMMOLASE heat-activation.
A 125 bp DNA fragment from plasmid pGEM was amplified with Taq (lanes 1-4) and IMMOLASE (lanes 5-8). The pGEM fragment was amplified from 0.25 ng DNA followed by 2-fold serial dilutions in 50 μL reactions. HyperLadder 25bp (M). Two tests were conducted, with hot-start and without hot-start. Taq exhibited activity in both tests, whereas IMMOLASE only exhibited activity following a hot-start step.
|
Product |
250 Units |
500 Units |
|
IMMOLASE DNA Polymerase |
1 x 50 µL |
1 x 100 µL |
|
10x ImmoBuffer |
1.2 mL |
2 x 1.2 mL |
|
50 mM MgCl2 Solution |
1.2 mL |
1 x 1.2 mL |
5 u/µL
All components should be stored at -20°C upon receipt for optimum stability. Repeated freeze/thaw cycles should be avoided. When stored under the recommended conditions and handled correctly, full activity of the reagents is retained until the expiry date indicated on the outer box label.
Shipped on Dry Ice or Blue Ice.
IMMOLASE DNA Polymerase requires a heat-activation step of 10 minutes at 95°C.
As well as most standard applications, IMMOLASE DNA Polymerase is ideally suited to the following:
- High-throughput applications
- Multiplex PCR
- TA Cloning
IMMOLASE DNA Polymerase has been manufactured under 13485 Quality Management System and is suitable for further manufacturing use as an IVD component.
The features of IMMOLASE DNA Polymerase are as follows:
Optimal Extension Temperature: 72°C
| Observation | Recommended Solution(s) |
| No or low PCR yield | Enzyme concentration too low – increase the amount of enzyme in 0.5 U increments. |
| Primers degraded – check quality and age of the primers. | |
| Magnesium concentration too low – increase concentration in 0.25 mM increments with a starting concentration of 1.75 mM. | |
| Primer concentration not optimized. Titrate primer concentration (0.3-1 µM); ensuring that both primers have the same concentration. | |
| Template concentration too low – Increase concentration of template. | |
| Perform a positive control to ensure that the enzyme, dNTPs and buffers are not degraded and/or contaminated. | |
| Multiple Bands | Primer annealing temperature too low. Increase annealing temperature. Primer annealing should be at least 5°C below the calculated Tm of primers. |
| Prepare master mixes on ice or use a heat-activated polymerase. | |
| For problems with low specificity. Try adding 3% DMSO (not supplied) to improve specificity. | |
| Smearing or artifacts | Template concentration too high. Prepare serial dilutions of template. |
| Too many cycles. Reduce the cycle number by 3-5 to remove non-specific bands. | |
| Enzyme concentration too high - decrease the amount of enzyme in 0.5 U increments. | |
| Extension time too long. Reduce extension time in 0.5-1 minute increments. |