Ordering
*link will take you to our exclusive distribution partner site
*link will take you to our exclusive distribution partner site
RNA was isolated from 20 mg freeze-dried budding leaves of A. thaliana using ISOLATE II RNA Plant Kit. The extracted RNA was diluted in a 2-fold serial dilution (lanes 1-7) and PCR was performed using MyTaq One-Step RT-PCR Kit. The results illustrate the quality of the RNA obtained, as it can be used for very sensitive cDNA synthesis and PCR without further purification.
The ISOLATE II RNA Plant Kit provides a simple, efficient column-based method for the isolation of total RNA from a wide variety of plant materials, including leaves, bark, roots and fruits, without the need for hazardous reagents such as phenol.
By combining the stringency of guanidinium thiocyanate lysis with the speed and ease-of-use of silica-membrane purification, the ISOLATE II RNA Plant Kit provides a fast method for the purification of high-quality total RNA from most plant cells, including plant tissues, leaves, bark, roots and fruits.
Some plant tissues such as maize endosperm and the mycelia of filamentous fungi do not lyse well in guanidinium thiocyanate and can solidify, resulting in poor yields and so guanidinium hydrochloride lysis buffer is also provided as an alternative. Residual amounts of contaminating DNA are selectively removed using a column, however for ultrasensitive applications, remaining DNA can be removed using RNase-free DNase I that is supplied with the kit.
The ISOLATE II RNA Plant Kit has been designed to deliver optimal performance in RT-qPCR in conjunction with either the SensiFAST cDNA Synthesis Kit and SensiFAST Real-Time PCR Kits, or the SensiFAST One-Step Real-Time RT-PCR Kits. Additionally, the ISOLATE II RNA Plant Kit can be used to purify samples prior to RT-PCR amplification using the Tetro cDNA Synthesis Kit and any enzyme from the Meridian PCR portfolio, including MyTaq DNA Polymerase.
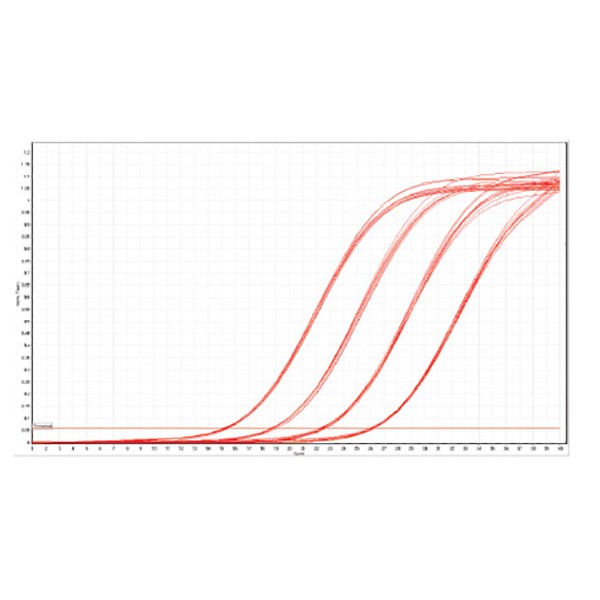
Superior performance in real-time applications
RNA was isolated from 20 mg freeze-dried budding leaves of A. thaliana using ISOLATE II RNA Plant Kit. The RNA was split into twelve replicates and diluted in a 10-fold series. Real-time reactions were performed using primers for a fragment of UBQ10 gene and SensiFAST SYBR No-ROX One-Step Kit. The results illustrate the quality of the RNA extracted through the reproducibility of the amplification.
High quality RNA isolated from plant tissue
RNA was isolated from 20 mg freeze-dried budding leaves of A. thaliana using ISOLATE II RNA Plant Kit. The extracted RNA was diluted in a 2-fold serial dilution (lanes 1-7) and PCR was performed using MyTaq One-Step RT-PCR Kit. The results illustrate the quality of the RNA obtained, as it can be used for very sensitive cDNA synthesis and PCR without further purification.
|
Reagent |
10 Preps |
50 Preps |
|
ISOLATE II Filters |
10 |
50 |
|
ISOLATE II RNA Mini Columns |
10 |
50 |
|
Collection Tubes (2 mL) |
30 |
150 |
|
Collection Tubes (1.5 mL) |
10 |
50 |
|
Lysis Buffer RLY |
10 mL |
25 mL |
|
Lysis Buffer RLS |
10 mL |
25 mL |
|
Wash Buffer RW1 |
15 mL |
15 mL |
|
Wash Buffer RW2 |
6 mL |
12 mL |
|
Membrane Desalting Buffer MEM |
10 mL |
25 mL |
|
Reaction Buffer for DNase I RDN |
7 mL |
7 mL |
|
DNase I, RNase-free (lyophilized) |
1 vial |
1 vial |
|
RNase-free water |
13 mL |
13 mL |
|
Bench Protocol Sheet |
1 |
1 |
All components should be stored dry and at room temperature.
When stored under the recommended conditions and handled correctly, full activity of reagents is retained until the expiry date indicated on the outer box label.
Ambient temperature.
Agarose gel analysis of RNA samples can be both valuable and misleading. The pattern of bands on the gel can not only be indicative of the quality of the sample, but equally it can also only indicate that the gel tank, buffer or agarose is contaminated with RNase. A safer measure is to use a Bioanalyzer and look at the RIN value (RNA Integrity Number), which should be as close to 10 as possible, indicating that the RNA is not degraded. A spectrophotometer (ideally a microfluidic one) can also be used to determine the ratio of A260 to A280 for purity determination.
All these methods give an idea of the quality of total RNA which tends to be dominated with rRNA and tRNA. It is not uncommon for an agarose gel to show an abundance of 18S and 23S rRNA but on analysis for there to be little of the transcript of interest. Ideally RNA quality control should include an assessment of the presence of common transcripts (from reference genes, such as GAPDH) using RT-PCR or RT-qPCR.
If the yield of RNA is low, it is best to first check that all the solutions used and the equipment employed is largely free of RNase. Solutions should be prepared with the highest quality reagents available, preferably ones that have a certificate of analysis to show that they are free of DNase and RNase. If there is confidence that RNase contamination is at a minimum, then it may be worth ensuring that the sample is properly homogenised before lysis and to check a sample of the lysed material under a light microscope to ensure that all the cells are disrupted.
RNA samples that have been contaminated with RNase (either during processing or those that have been sent from another lab) can be purified using the ISOLATE II RNA Micro Kit.
The lysis buffer RLY and RLS contain guanidinium thiocyanate and guanidinium hydrochloride, respectively. Lysis buffer RLY works in most cases and is recommended for the lysis of most plant materials due to the stronger denaturation properties. However, some plant tissues or fungi solidify in RLY and RNA purification cannot proceed, buffer RLS should be used instead.