Ordering
*link will take you to our exclusive distribution partner site
*link will take you to our exclusive distribution partner site
MangoTaq™ DNA Polymerase is a formulation of Taq DNA Polymerase that offers high-yield across a wide range of DNA concentrations.
MangoTaq DNA Polymerase possesses 5´-3´ exonuclease activity and leaves an ´A´ overhang, resulting in PCR product suitable for effective integration into TA cloning vectors.
MangoTaq™ DNA Polymerase offers high yield across a wide range of DNA concentrations. MangoTaq DNA Polymerase leaves an ´A´ overhang such that the PCR product is suitable for effective integration into TA cloning vectors.
The polymerase is supplied with two different reaction buffers for greater flexibility. For high-throughput applications, MangoTaq and the colored reaction buffer make an ideal choice, since this combination enables the user to load directly on a gel in order to facilitate easy recognition.
The two reaction buffers supplied are: 5x Colored Reaction Buffer and 5x Colorless Reaction Buffer. The colored reaction buffer contains red and orange dyes, which separate during electrophoresis and provide quick reference points for monitoring the mobility of the DNA samples in the gel. The colored reaction buffer can be loaded directly onto an agarose gel for analysis without the need for separate gel-loading buffer. The presence of the dyes has no effect on routine enzymatic manipulations, although extremely rare exceptions may exist.
Since the colorless reaction buffer does not contain reference dyes, it is suitable for use when reaction products will be used directly for down-stream processes involving absorbance or fluorescent detection. The specificity and performance of MangoTaq DNA Polymerase can be further improved with the use of 3% DMSO, which is designed for GC or AT-rich DNA, "dirty" templates or sequences with a high level of secondary structure.
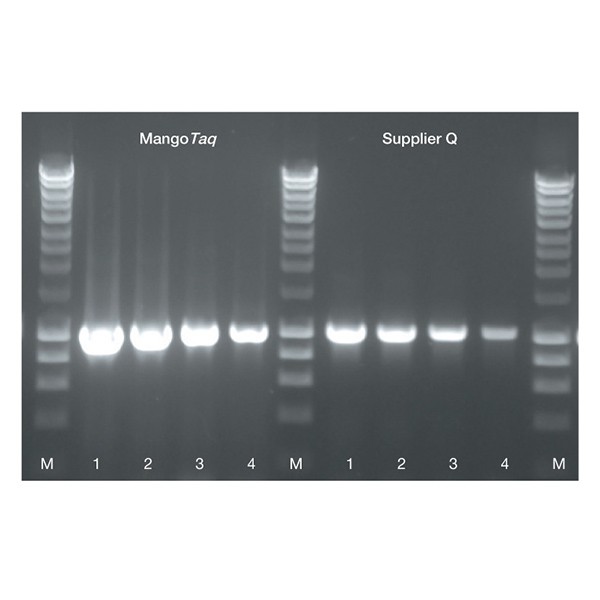
Fig. 1 Amplification of a 1 kb fragment from l DNA using MangoTaq DNA Polymerase and supplier Q Taq DNA Polymerases.
A 1 kb l DNA fragment was amplified using MangoTaq and Taq from supplier Q. The DNA fragment was amplified from a 5-fold serial dilution of l DNA with an initial concentration of 0.5 ng (lane 1), 0.1 ng l DNA (lane 2), 0.02 ng lDNA (lane 3), 4 pg l DNA (lane 4). PCR was performed in 50 µL reaction mixtures containing 1.5 mM MgCl2. HyperLadder 1kb (M).
Fig. 2 Amplification of a range of fragments from different human genes using MangoTaq DNA Polymerase and supplier Q Taq DNA Polymerase.
The amplification products are as follows: 119 bp (43% GC) from human glucocerebrosidase gene (1), 321 bp (37% GC) from angiotensin receptor II gene (2), 635 bp (56% GC) from rhodopsin gene (3), 762 bp (33% GC) from b-globin gene (4), 1200 bp (54% GC) from a-1-antitrypsin gene (5). PCR was performed in 50 µL reaction mixtures containing 50 ng human genomic DNA and 1.5 mM MgCl2. HyperLadder 50bp (M).
|
Reagent |
1000 Units |
|
MangoTaq |
200 μL |
|
5x MangoTaq Colored Reaction Buffer |
4 x 1.5 mL |
|
5x MangoTaq Colorless Reaction Buffer |
4 x 1.5 mL |
|
50 mM MgCl2 Solution |
2 x 1.2 mL |
5 u/µL
All components should be stored at -20°C upon receipt for optimum stability. Repeated freeze/thaw cycles should be avoided.
When stored under the recommended conditions and handled correctly, full activity of the reagents is retained until the expiry date indicated on the outer box label.
On Dry Ice or Blue Ice.
One unit will incorporate 10 nmoles of dNTPs in 30 min at 72°C
| Observation | Recommended Solution(s) |
| No or low PCR yield | Enzyme concentration too low – increase the amount of enzyme in 0.5 U increments. |
| Primers degraded – check quality and age of the primers. | |
| Magnesium concentration too low – increase concentration in 0.25 mM increments with a starting concentration of 1.75 mM. | |
| Primer concentration not optimized. Titrate primer concentration (0.3-1 µM); ensuring that both primers have the same concentration. | |
| Template concentration too low – Increase concentration of template. | |
| Perform a positive control to ensure that the enzyme, dNTPs and buffers are not degraded and/or contaminated. | |
| Multiple Bands | Primer annealing temperature too low. Increase annealing temperature. Primer annealing should be at least 5°C below the calculated Tm of primers. |
| Prepare master mixes on ice or use a heat-activated polymerase. | |
| For problems with low specificity. Try adding 3% DMSO (not supplied) to improve specificity. | |
| Smearing or artifacts | Template concentration too high. Prepare serial dilutions of template. |
| Too many cycles. Reduce the cycle number by 3-5 to remove non-specific bands. | |
| Enzyme concentration too high - decrease the amount of enzyme in 0.5 U increments. | |
| Extension time too long. Reduce extension time in 0.5-1 minute increments. |